Mercury »
PDB 1is9-1obh »
1nu7 »
Mercury in PDB 1nu7: Staphylocoagulase-Thrombin Complex
Enzymatic activity of Staphylocoagulase-Thrombin Complex
All present enzymatic activity of Staphylocoagulase-Thrombin Complex:
3.4.21.5;
3.4.21.5;
Protein crystallography data
The structure of Staphylocoagulase-Thrombin Complex, PDB code: 1nu7
was solved by
R.Friedrich,
W.Bode,
P.Fuentes-Prior,
P.Panizzi,
P.E.Bock,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 14.99 / 2.20 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 180.170, 102.000, 135.300, 90.00, 130.08, 90.00 |
| R / Rfree (%) | 20.9 / 24.9 |
Mercury Binding Sites:
The binding sites of Mercury atom in the Staphylocoagulase-Thrombin Complex
(pdb code 1nu7). This binding sites where shown within
5.0 Angstroms radius around Mercury atom.
In total 4 binding sites of Mercury where determined in the Staphylocoagulase-Thrombin Complex, PDB code: 1nu7:
Jump to Mercury binding site number: 1; 2; 3; 4;
In total 4 binding sites of Mercury where determined in the Staphylocoagulase-Thrombin Complex, PDB code: 1nu7:
Jump to Mercury binding site number: 1; 2; 3; 4;
Mercury binding site 1 out of 4 in 1nu7
Go back to
Mercury binding site 1 out
of 4 in the Staphylocoagulase-Thrombin Complex
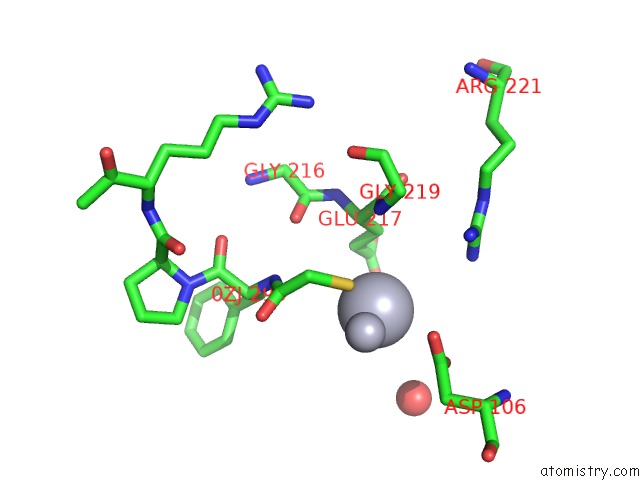
Mono view
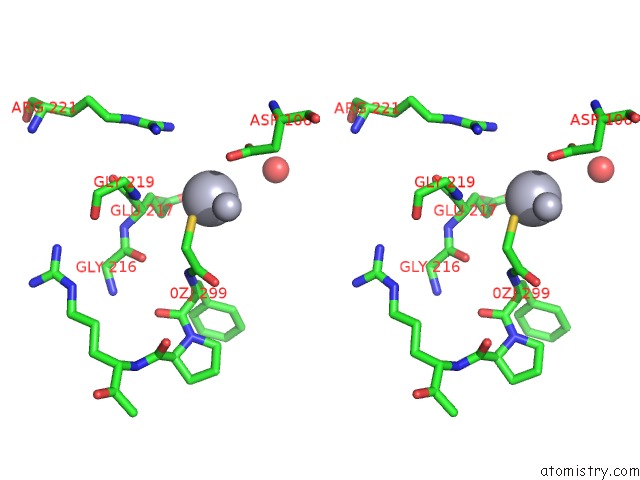
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 1 of Staphylocoagulase-Thrombin Complex within 5.0Å range:
|
Mercury binding site 2 out of 4 in 1nu7
Go back to
Mercury binding site 2 out
of 4 in the Staphylocoagulase-Thrombin Complex
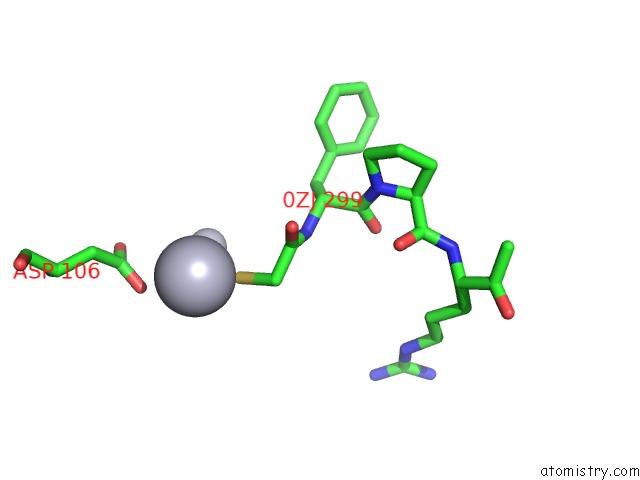
Mono view
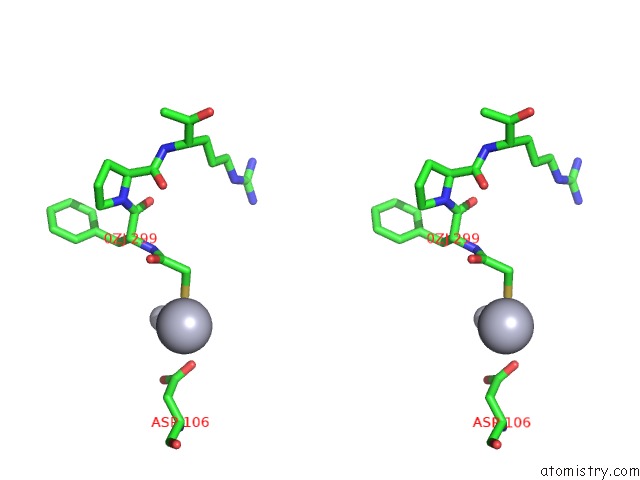
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 2 of Staphylocoagulase-Thrombin Complex within 5.0Å range:
|
Mercury binding site 3 out of 4 in 1nu7
Go back to
Mercury binding site 3 out
of 4 in the Staphylocoagulase-Thrombin Complex
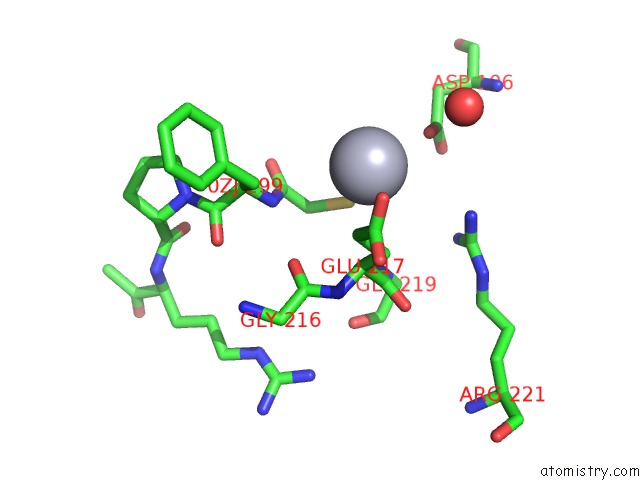
Mono view
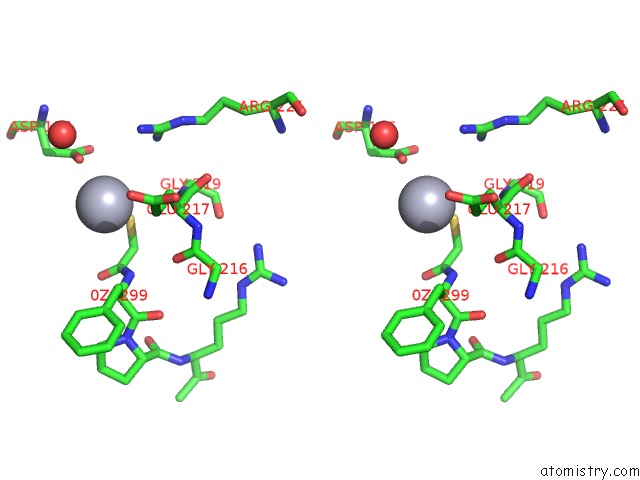
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 3 of Staphylocoagulase-Thrombin Complex within 5.0Å range:
|
Mercury binding site 4 out of 4 in 1nu7
Go back to
Mercury binding site 4 out
of 4 in the Staphylocoagulase-Thrombin Complex
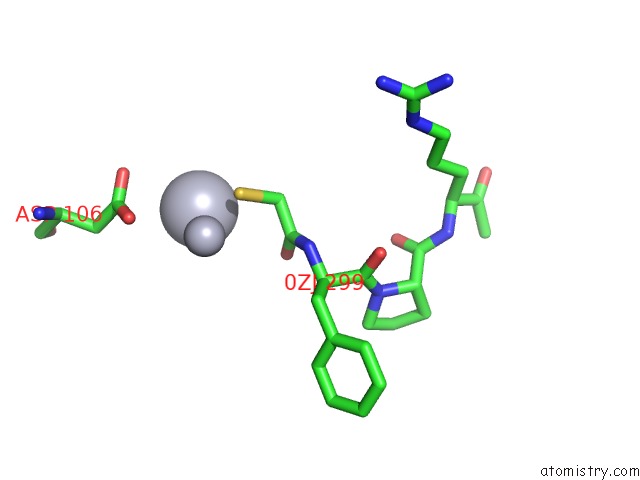
Mono view
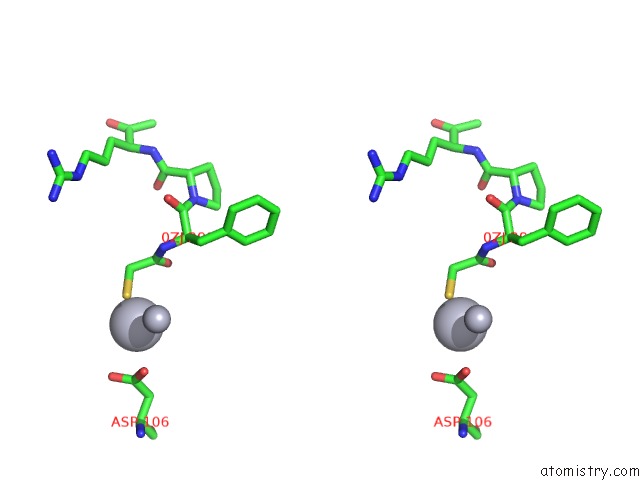
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 4 of Staphylocoagulase-Thrombin Complex within 5.0Å range:
|
Reference:
R.Friedrich,
P.Panizzi,
P.Fuentes-Prior,
K.Richter,
I.Verhamme,
P.J.Anderson,
S.Kawabata,
R.Huber,
W.Bode,
P.E.Bock.
Staphylocoagulase Is A Prototype For the Mechanism of Cofactor-Induced Zymogen Activation Nature V. 425 535 2003.
ISSN: ISSN 0028-0836
PubMed: 14523451
DOI: 10.1038/NATURE01962
Page generated: Fri Aug 8 09:18:34 2025
ISSN: ISSN 0028-0836
PubMed: 14523451
DOI: 10.1038/NATURE01962
Last articles
K in 7QQUK in 7QQV
K in 7QQT
K in 7QQR
K in 7QQS
K in 7QQQ
K in 7QQP
K in 7QQO
K in 7QK5
K in 7QIX