Mercury »
PDB 3kbc-3wa8 »
3kne »
Mercury in PDB 3kne: Carbonic Anhydrase II H64C Mutant in Complex with An in Situ Formed Triazole
Enzymatic activity of Carbonic Anhydrase II H64C Mutant in Complex with An in Situ Formed Triazole
All present enzymatic activity of Carbonic Anhydrase II H64C Mutant in Complex with An in Situ Formed Triazole:
4.2.1.1;
4.2.1.1;
Protein crystallography data
The structure of Carbonic Anhydrase II H64C Mutant in Complex with An in Situ Formed Triazole, PDB code: 3kne
was solved by
J.Schulze-Wischeler,
A.Heine,
G.Klebe,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 10.00 / 1.35 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 42.400, 41.500, 72.000, 90.00, 104.30, 90.00 |
| R / Rfree (%) | 12.8 / 18.3 |
Other elements in 3kne:
The structure of Carbonic Anhydrase II H64C Mutant in Complex with An in Situ Formed Triazole also contains other interesting chemical elements:
| Zinc | (Zn) | 1 atom |
Mercury Binding Sites:
The binding sites of Mercury atom in the Carbonic Anhydrase II H64C Mutant in Complex with An in Situ Formed Triazole
(pdb code 3kne). This binding sites where shown within
5.0 Angstroms radius around Mercury atom.
In total only one binding site of Mercury was determined in the Carbonic Anhydrase II H64C Mutant in Complex with An in Situ Formed Triazole, PDB code: 3kne:
In total only one binding site of Mercury was determined in the Carbonic Anhydrase II H64C Mutant in Complex with An in Situ Formed Triazole, PDB code: 3kne:
Mercury binding site 1 out of 1 in 3kne
Go back to
Mercury binding site 1 out
of 1 in the Carbonic Anhydrase II H64C Mutant in Complex with An in Situ Formed Triazole
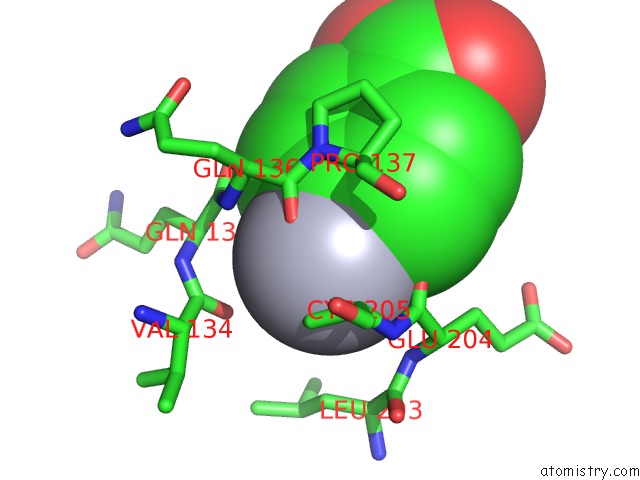
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 1 of Carbonic Anhydrase II H64C Mutant in Complex with An in Situ Formed Triazole within 5.0Å range:
|
Reference:
J.Schulze Wischeler,
D.Sun,
N.U.Sandner,
U.Linne,
A.Heine,
U.Koert,
G.Klebe.
Stereo- and Regioselective Azide/Alkyne Cycloadditions in Carbonic Anhydrase II Via Tethering, Monitored By Crystallography and Mass Spectrometry. Chemistry V. 17 5842 2011.
ISSN: ISSN 0947-6539
PubMed: 21506176
DOI: 10.1002/CHEM.201002437
Page generated: Fri Aug 8 10:12:08 2025
ISSN: ISSN 0947-6539
PubMed: 21506176
DOI: 10.1002/CHEM.201002437
Last articles
I in 2OW0I in 2OHU
I in 2OHT
I in 2OHS
I in 2OHN
I in 2OHR
I in 2OHQ
I in 2OHP
I in 2OHK
I in 2OHL