Mercury »
PDB 3wee-4ia4 »
3zzf »
Mercury in PDB 3zzf: Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate
Enzymatic activity of Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate
All present enzymatic activity of Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate:
2.7.2.8;
2.7.2.8;
Protein crystallography data
The structure of Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate, PDB code: 3zzf
was solved by
S.De Cima,
F.Gil-Ortiz,
M.Crabeel,
I.Fita,
V.Rubio,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 20.00 / 2.20 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 69.701, 99.299, 190.644, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 17.87 / 21.812 |
Other elements in 3zzf:
The structure of Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate also contains other interesting chemical elements:
| Chlorine | (Cl) | 7 atoms |
Mercury Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 11;Binding sites:
The binding sites of Mercury atom in the Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate (pdb code 3zzf). This binding sites where shown within 5.0 Angstroms radius around Mercury atom.In total 11 binding sites of Mercury where determined in the Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate, PDB code: 3zzf:
Jump to Mercury binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Mercury binding site 1 out of 11 in 3zzf
Go back to
Mercury binding site 1 out
of 11 in the Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate
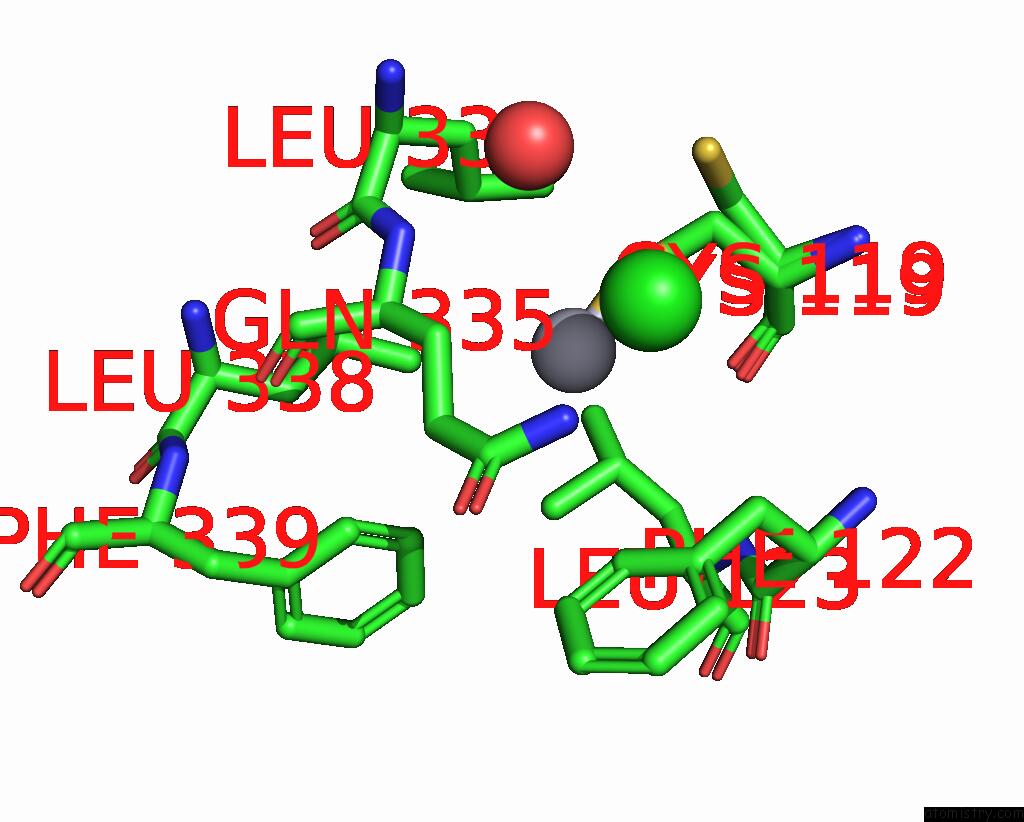
Mono view
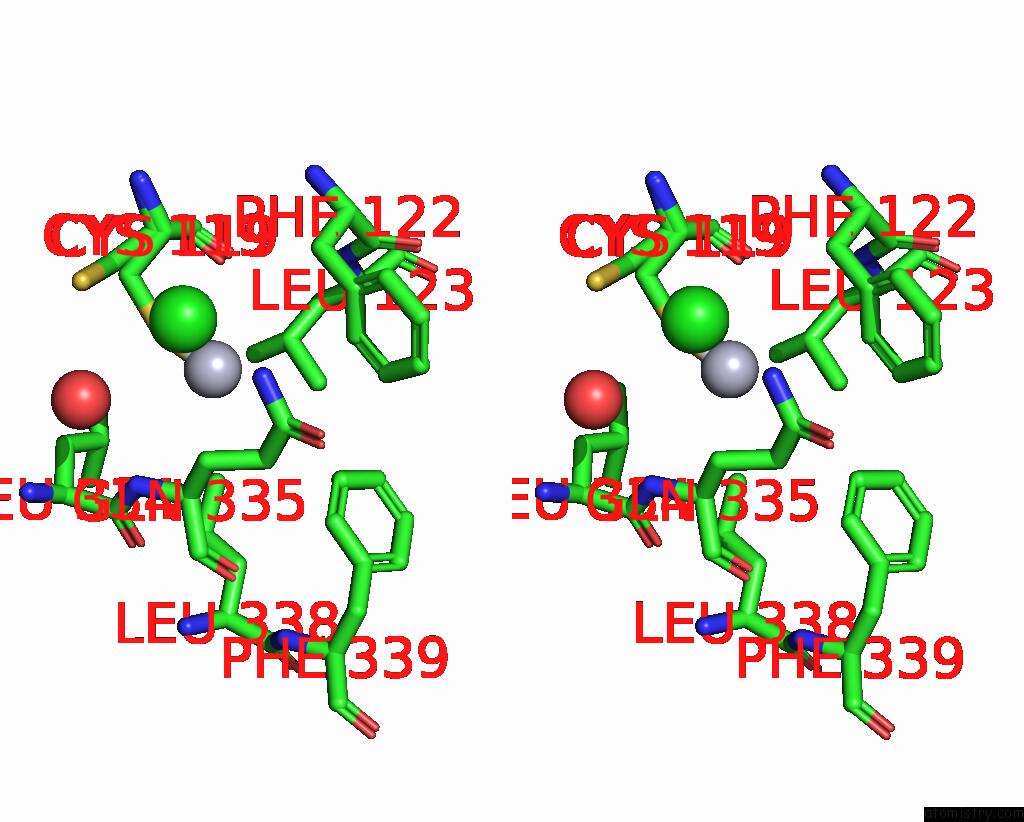
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 1 of Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate within 5.0Å range:
|
Mercury binding site 2 out of 11 in 3zzf
Go back to
Mercury binding site 2 out
of 11 in the Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate
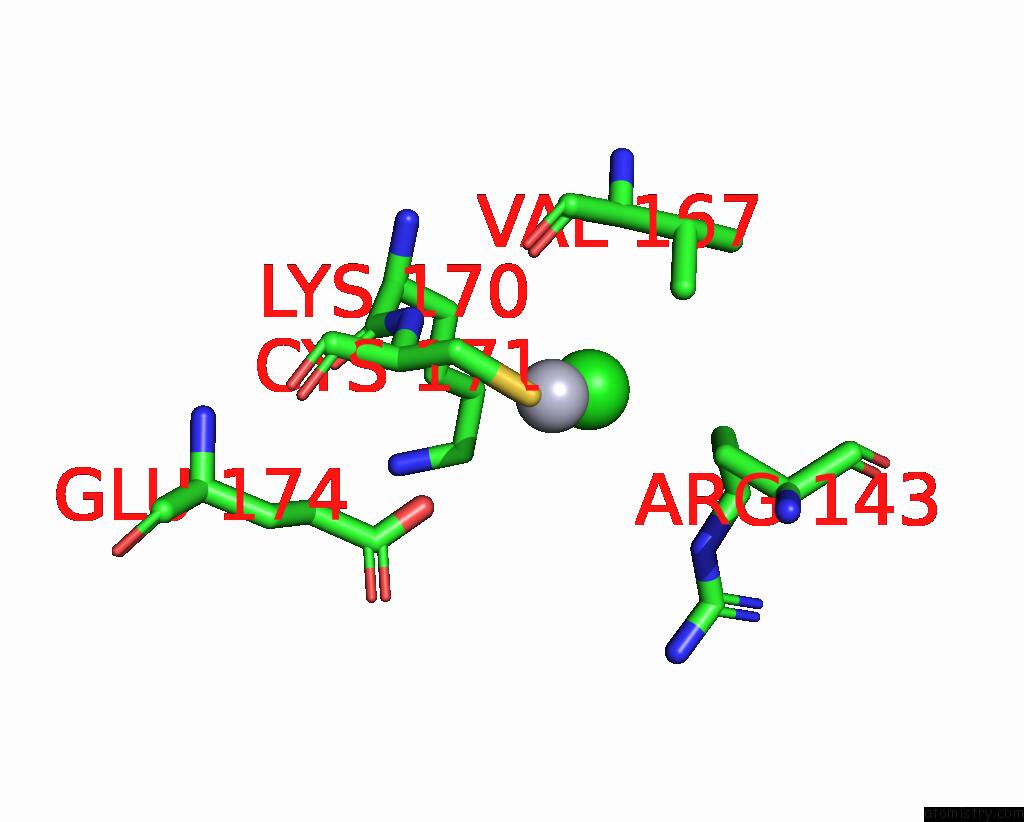
Mono view
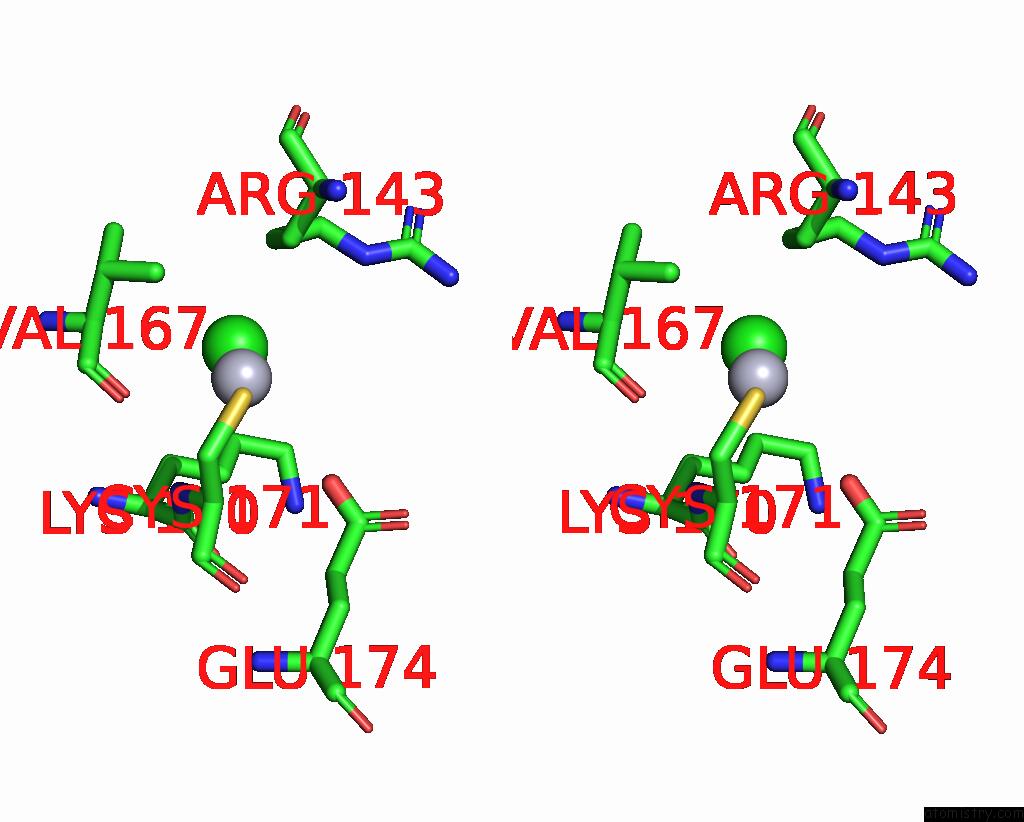
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 2 of Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate within 5.0Å range:
|
Mercury binding site 3 out of 11 in 3zzf
Go back to
Mercury binding site 3 out
of 11 in the Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate
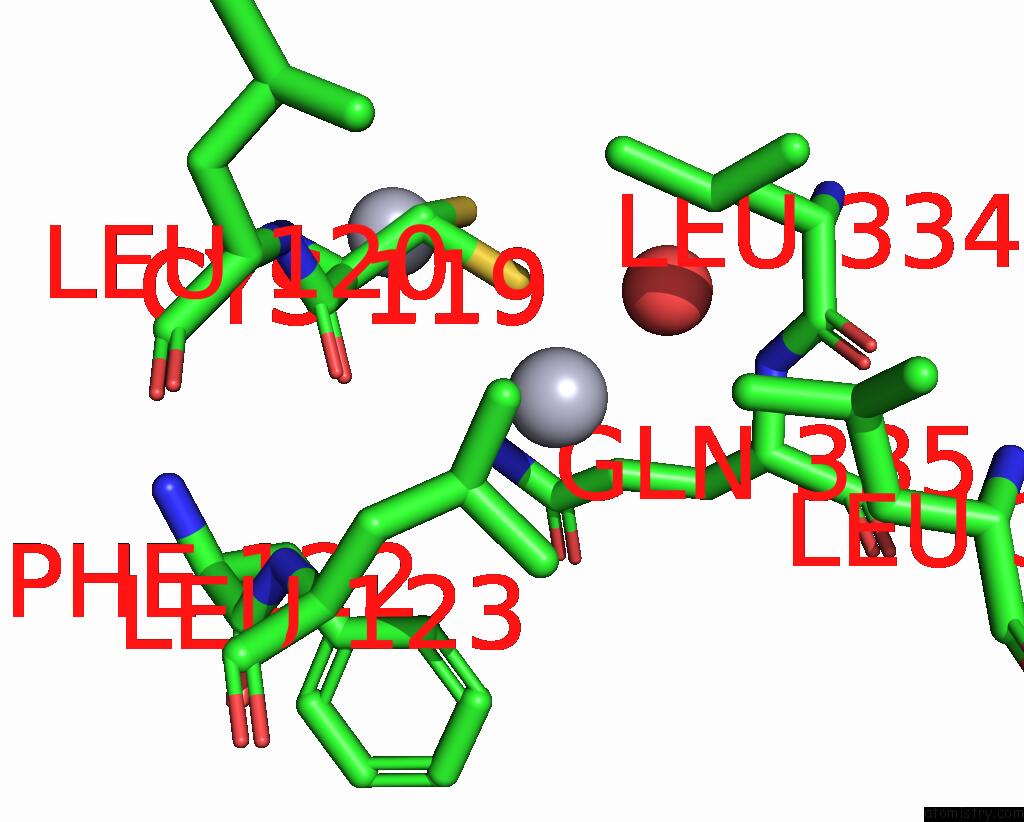
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 3 of Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate within 5.0Å range:
|
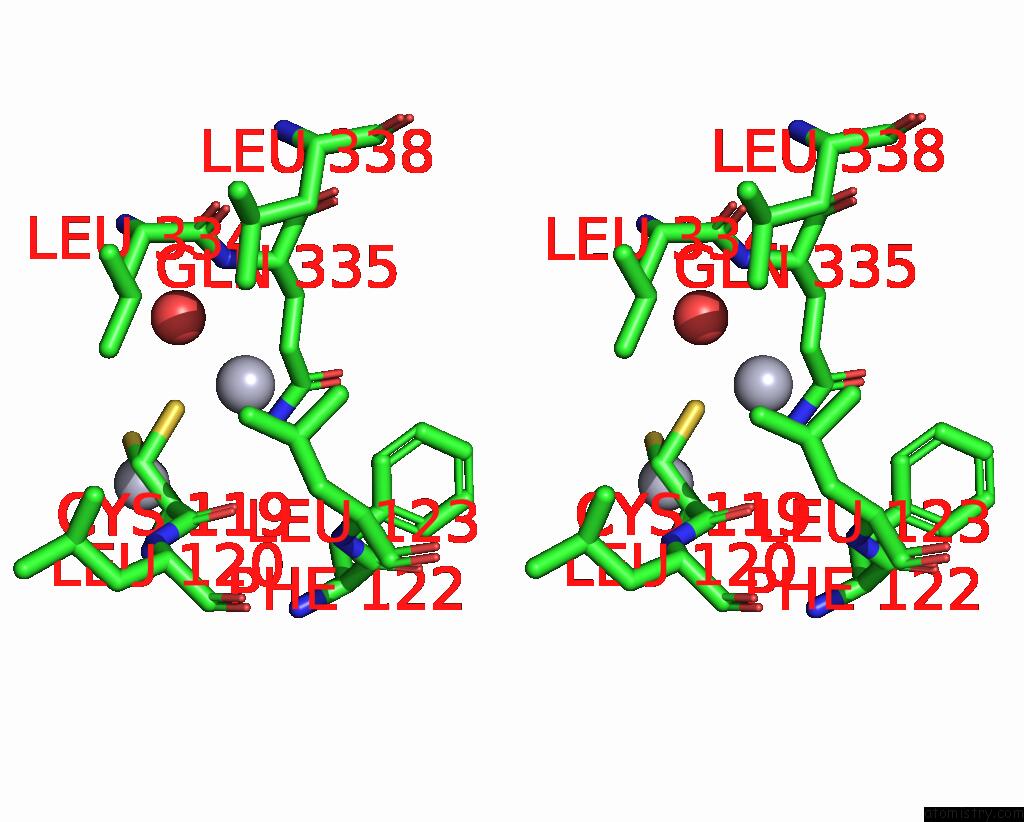
Mercury binding site 4 out of 11 in 3zzf
Go back to
Mercury binding site 4 out
of 11 in the Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate
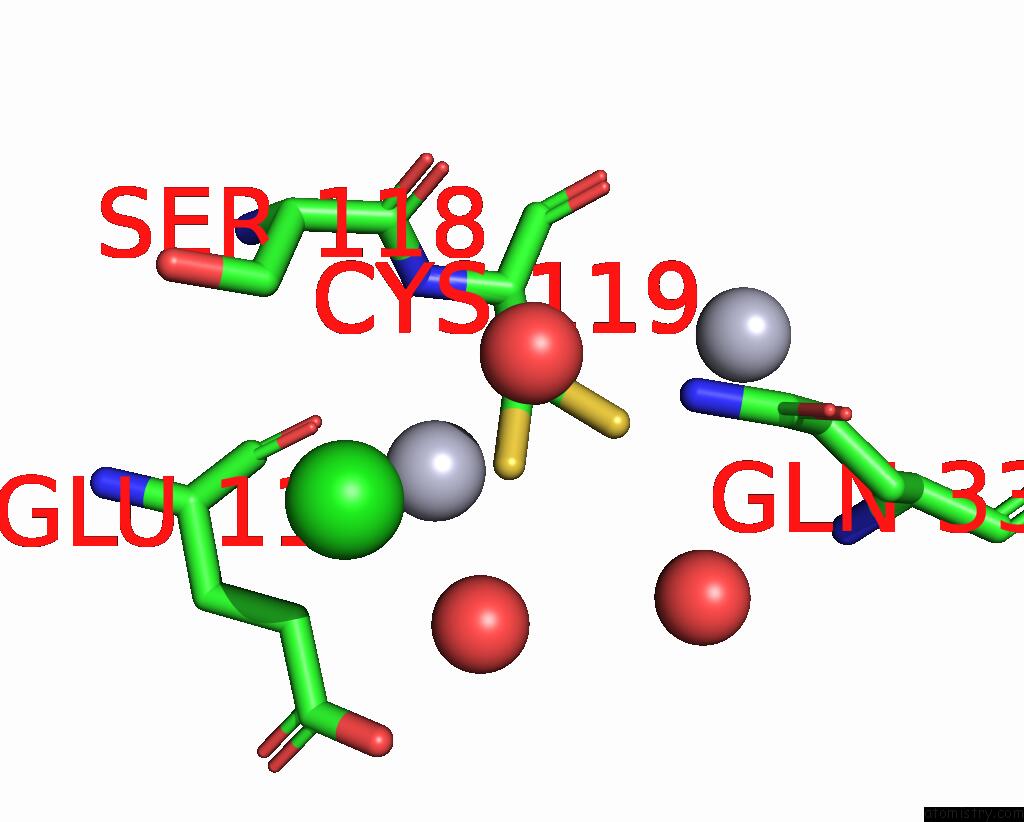
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 4 of Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate within 5.0Å range:
|
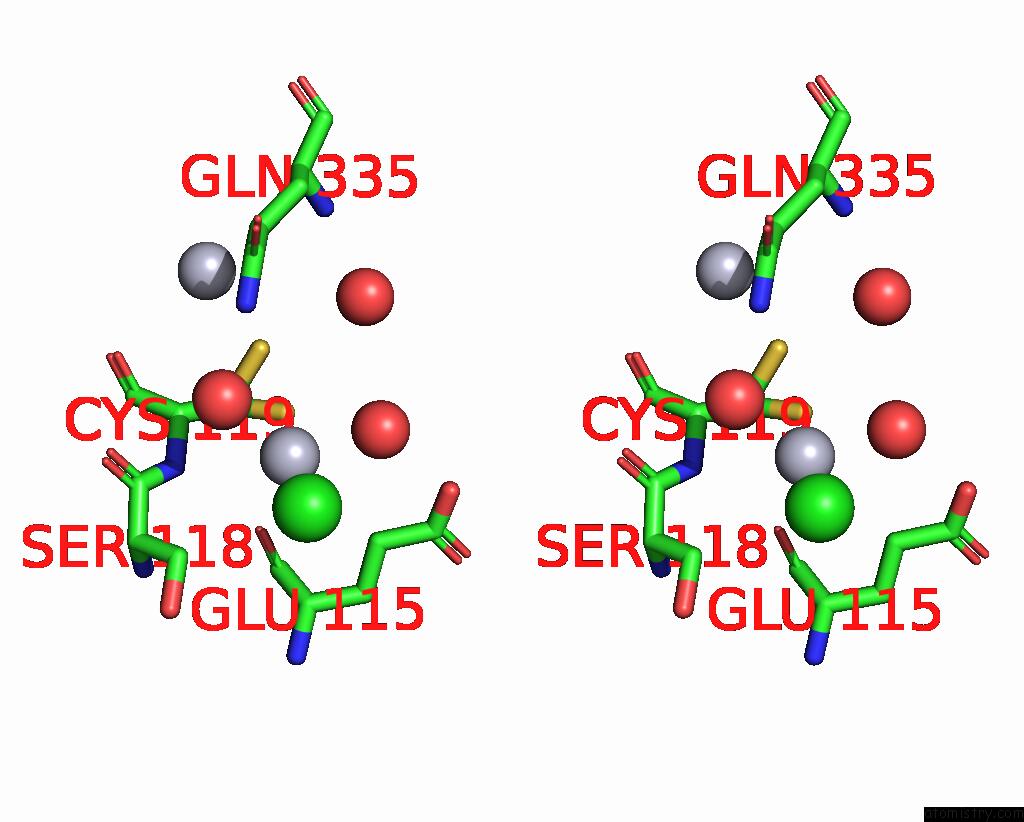
Mercury binding site 5 out of 11 in 3zzf
Go back to
Mercury binding site 5 out
of 11 in the Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate
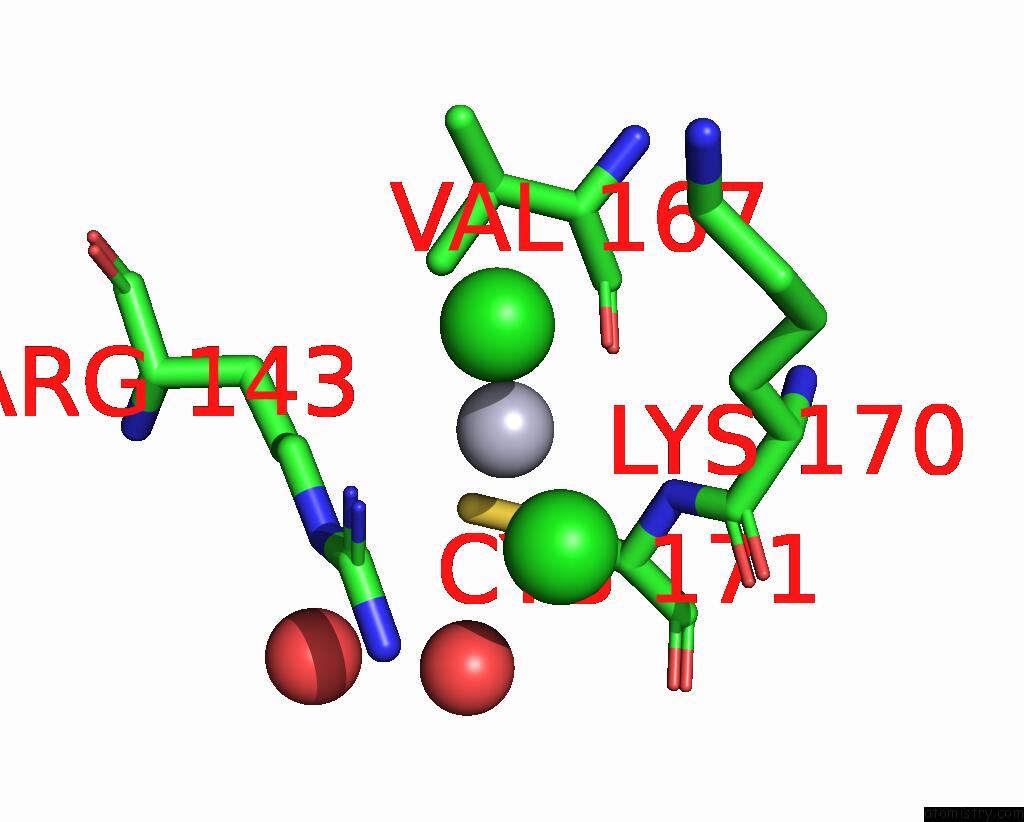
Mono view
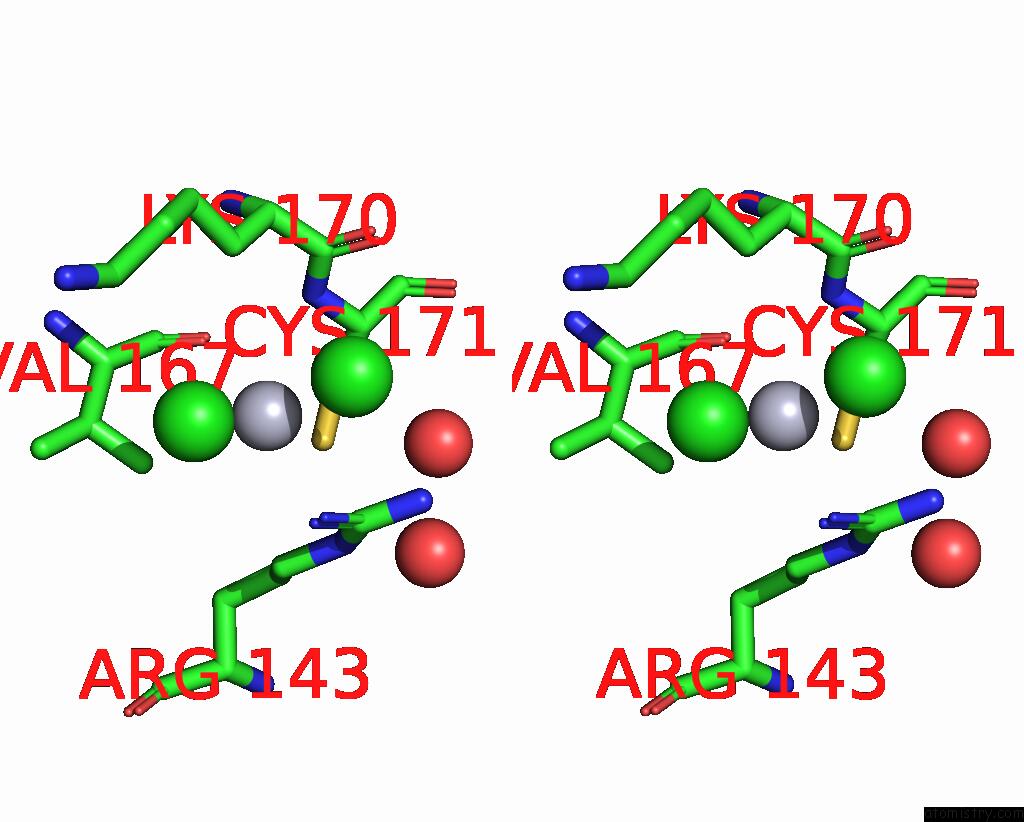
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 5 of Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate within 5.0Å range:
|
Mercury binding site 6 out of 11 in 3zzf
Go back to
Mercury binding site 6 out
of 11 in the Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate
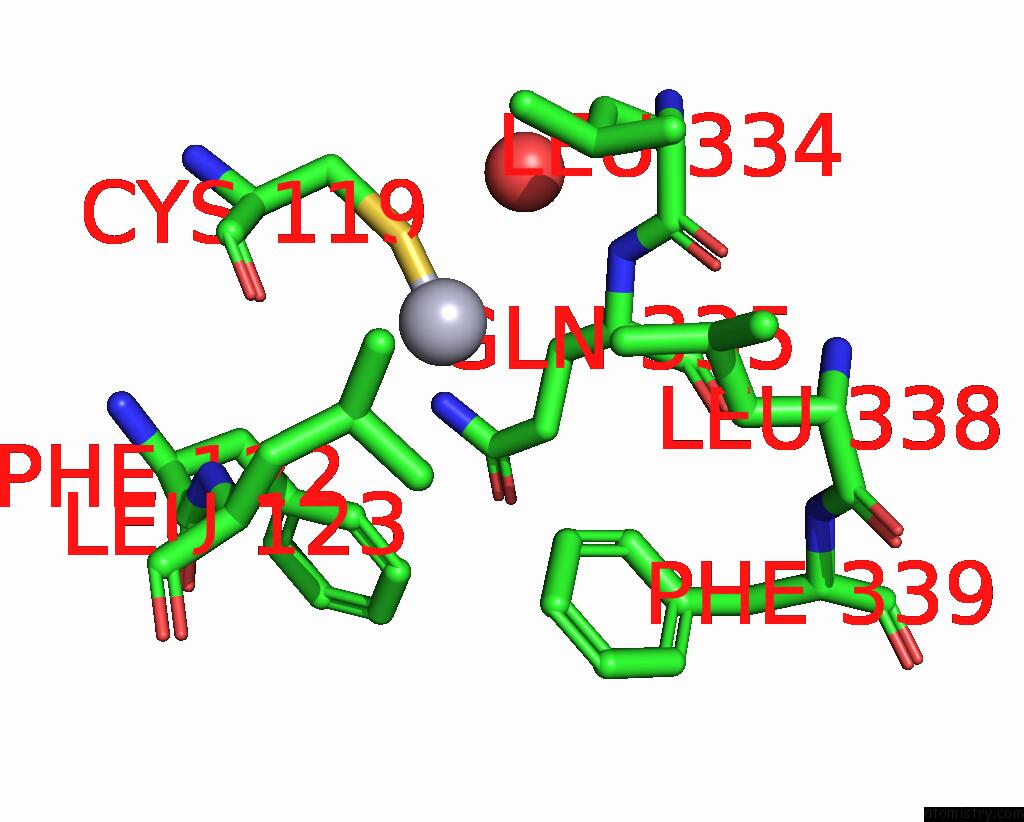
Mono view
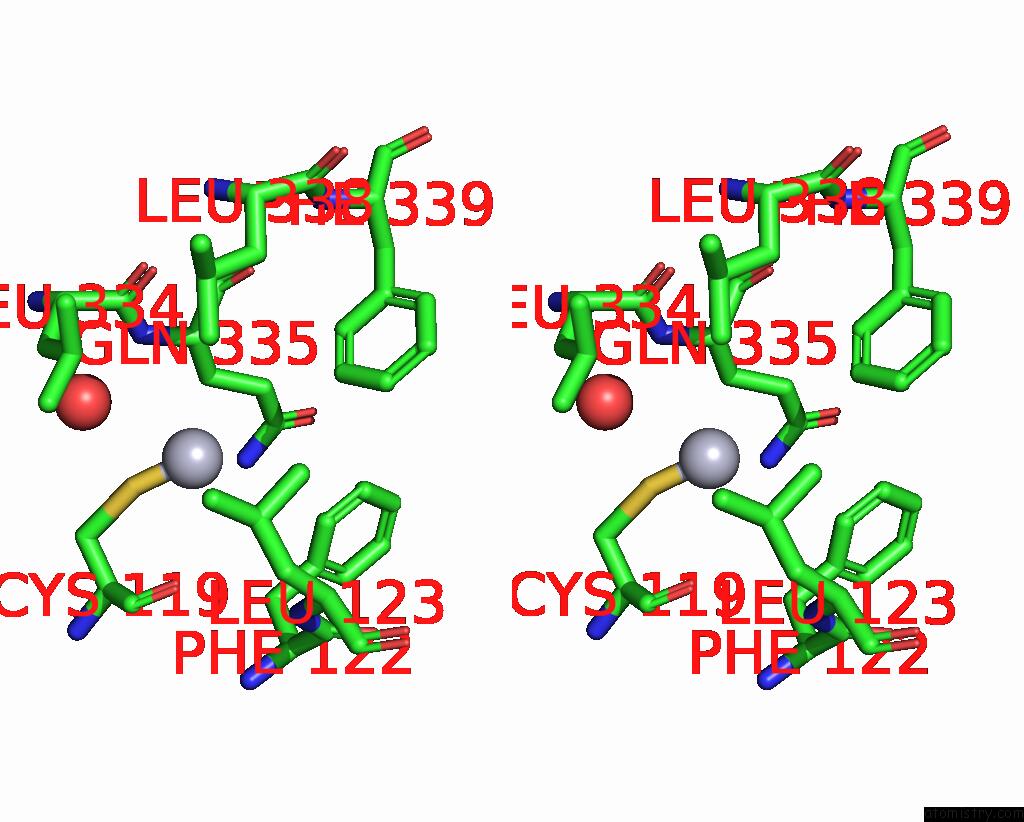
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 6 of Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate within 5.0Å range:
|
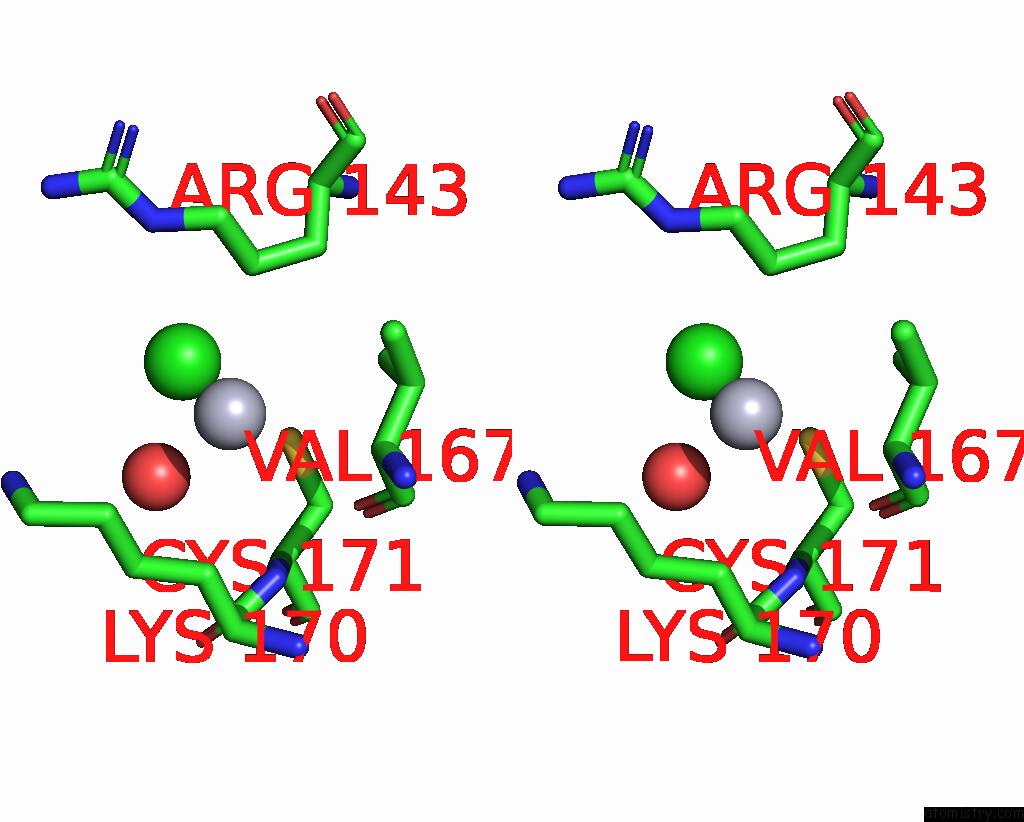
Mercury binding site 7 out of 11 in 3zzf
Go back to
Mercury binding site 7 out
of 11 in the Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate
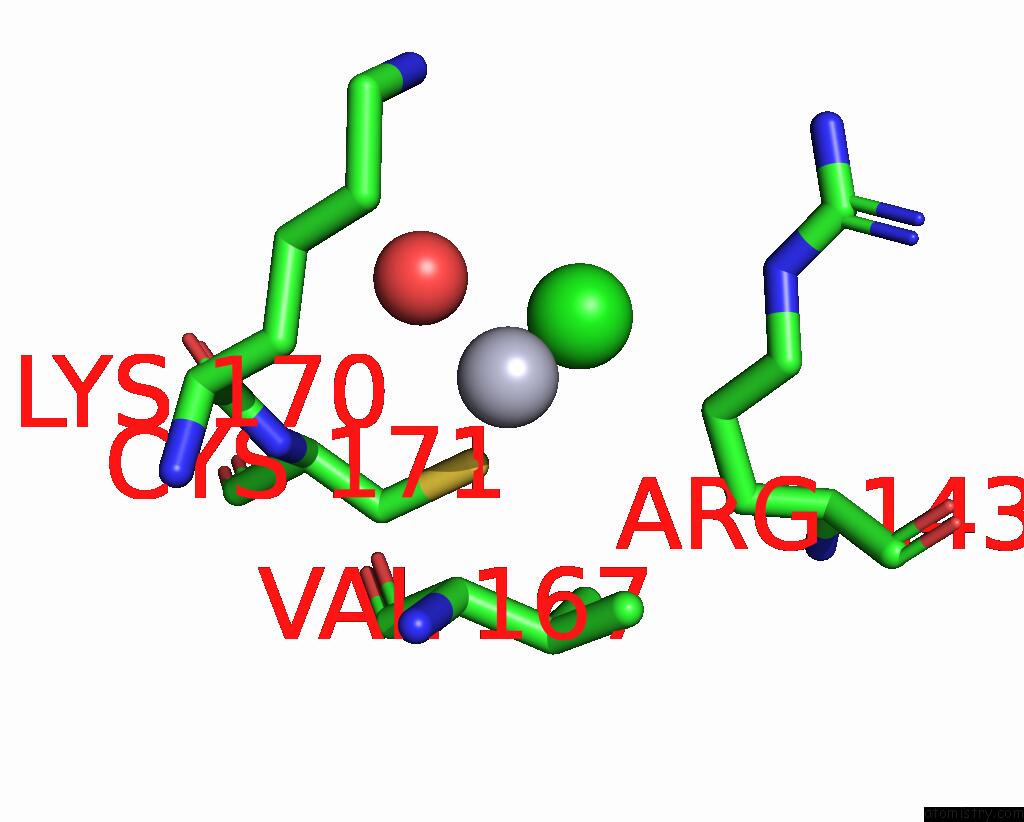
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 7 of Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate within 5.0Å range:
|
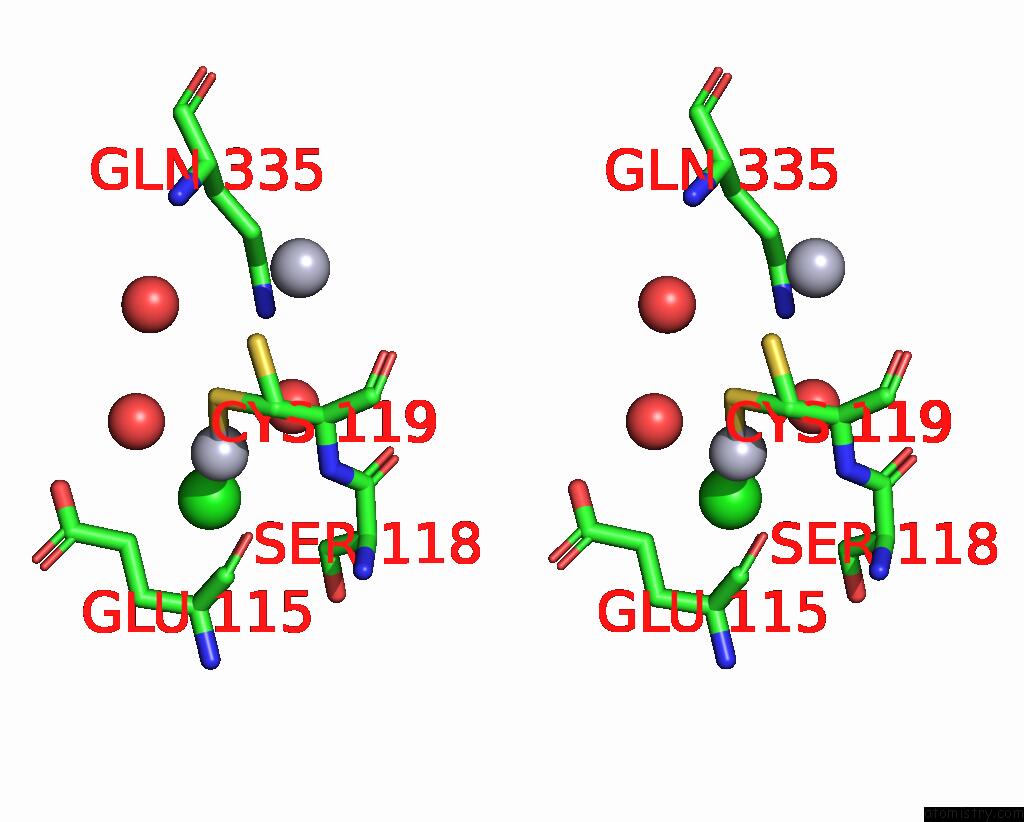
Mercury binding site 8 out of 11 in 3zzf
Go back to
Mercury binding site 8 out
of 11 in the Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate
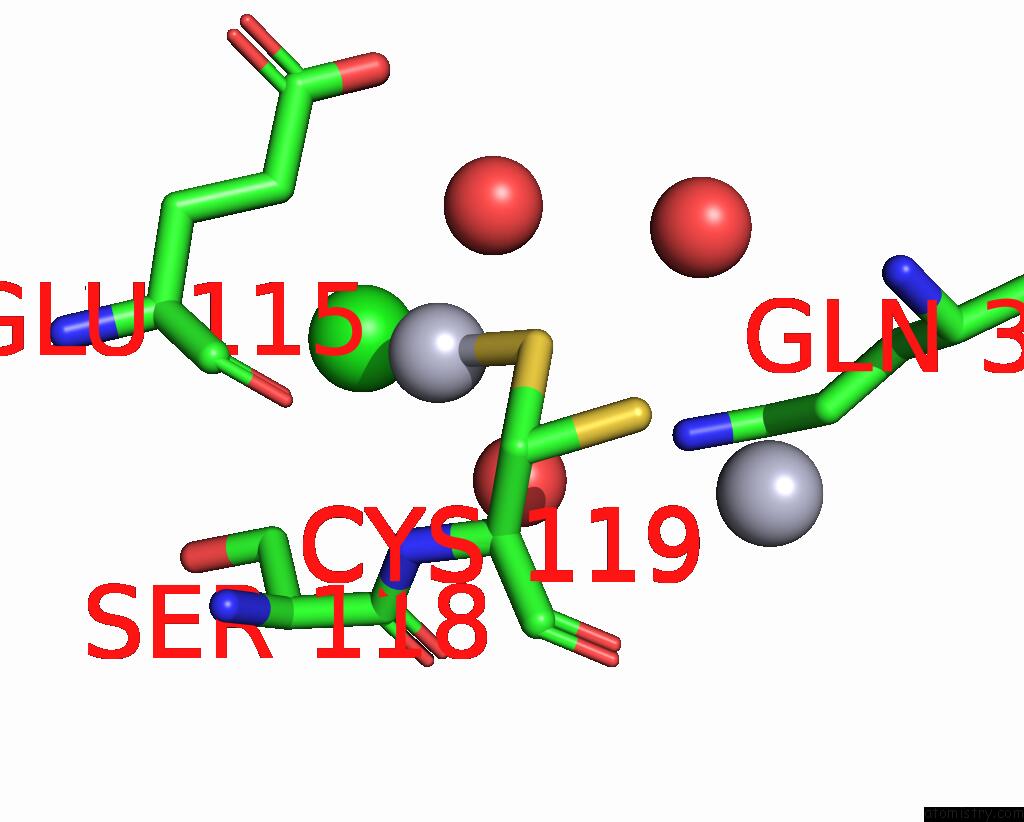
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 8 of Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate within 5.0Å range:
|
Mercury binding site 9 out of 11 in 3zzf
Go back to
Mercury binding site 9 out
of 11 in the Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate
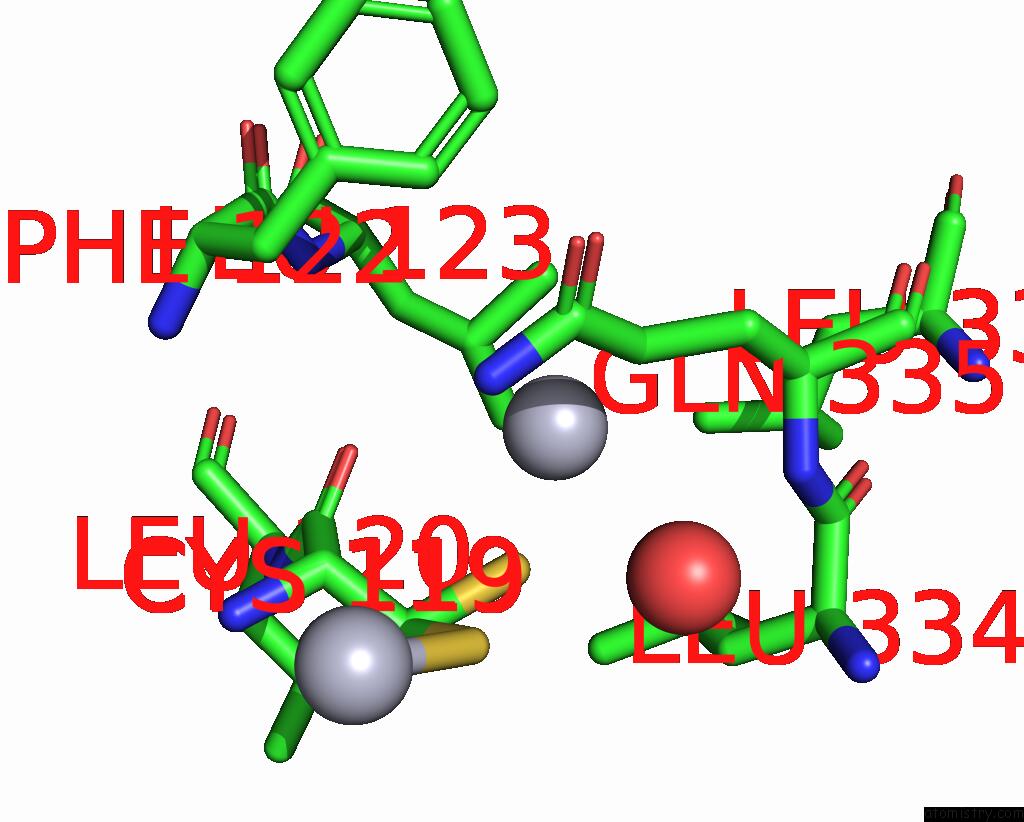
Mono view
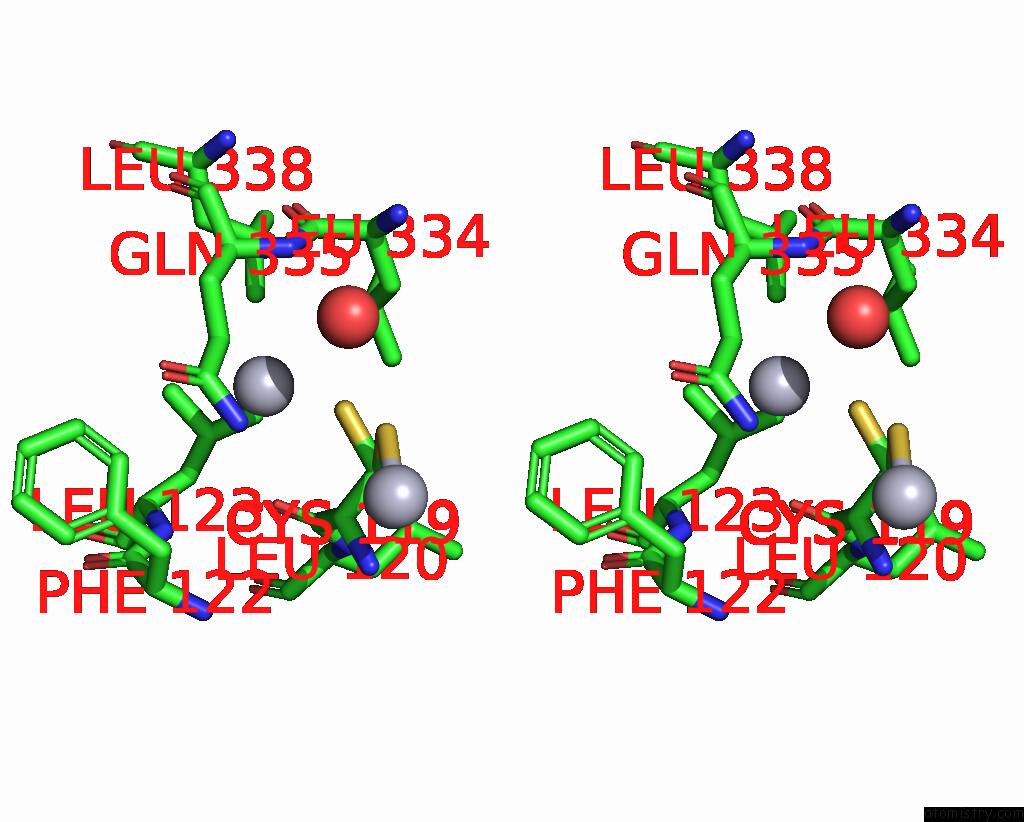
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 9 of Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate within 5.0Å range:
|
Mercury binding site 10 out of 11 in 3zzf
Go back to
Mercury binding site 10 out
of 11 in the Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate
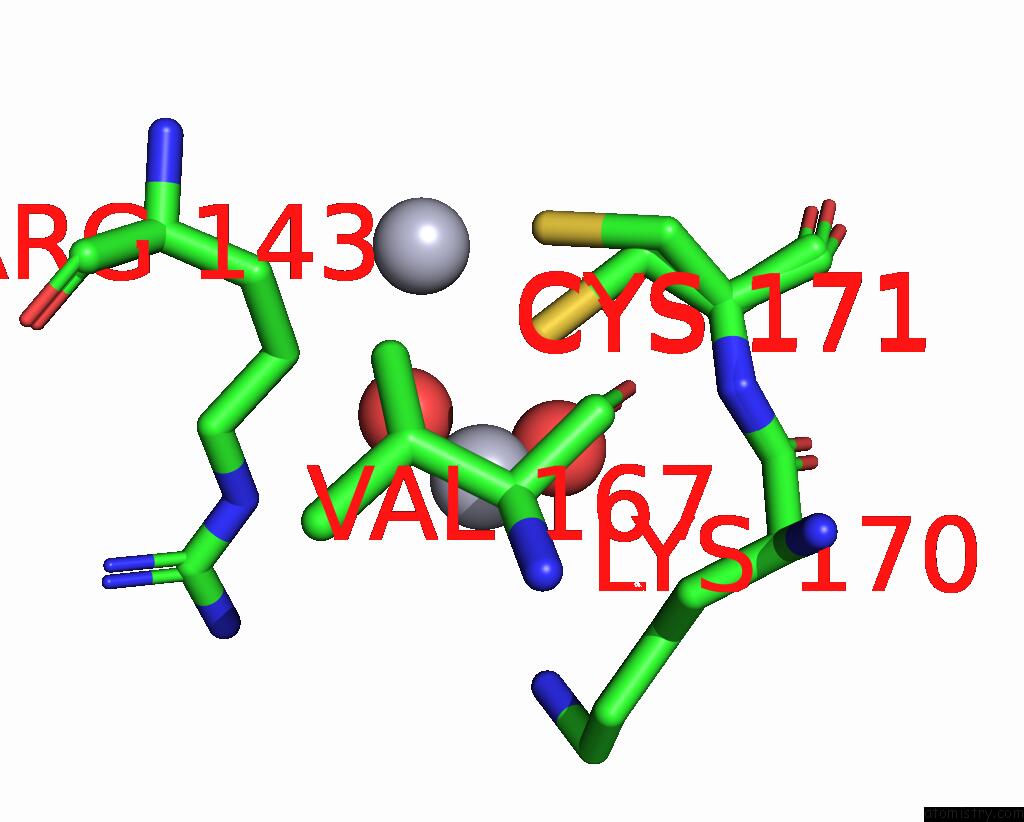
Mono view
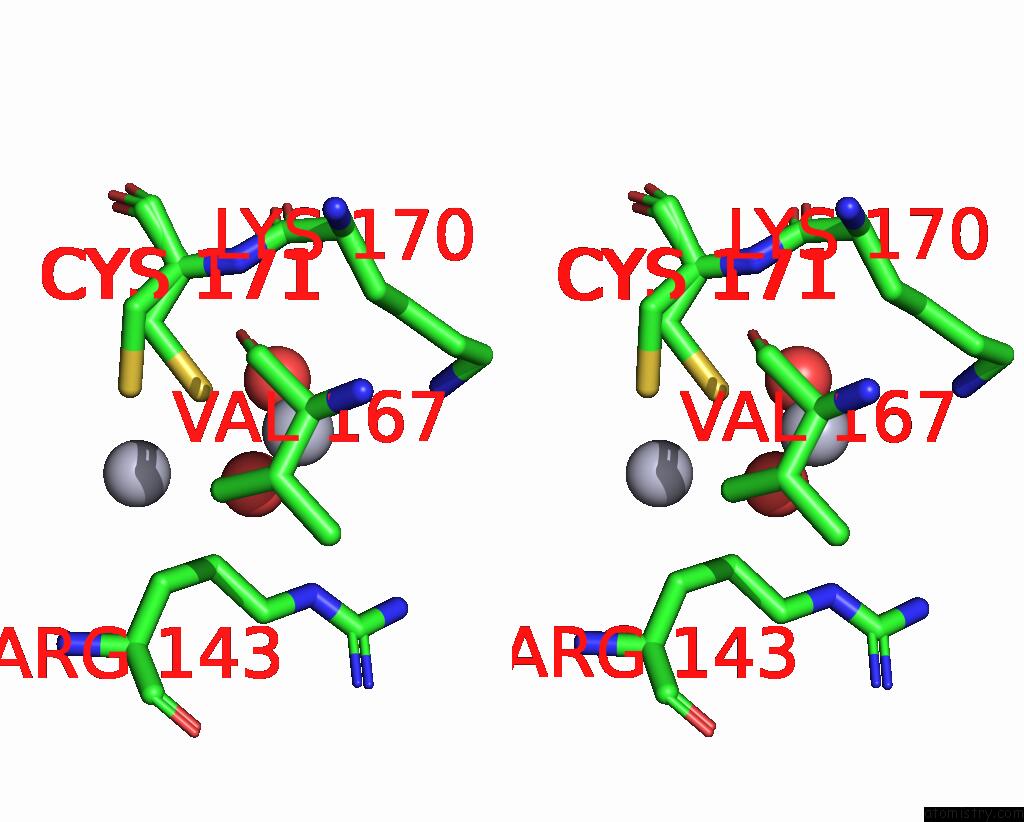
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 10 of Crystal Structure of the Amino Acid Kinase Domain From Saccharomyces Cerevisiae Acetylglutamate Kinase Complexed with Its Substrate N-Acetylglutamate within 5.0Å range:
|
Reference:
S.De Cima,
F.Gil-Ortiz,
M.Crabeel,
I.Fita,
V.Rubio.
Insight on An Arginine Synthesis Metabolon From the Tetrameric Structure of Yeast Acetylglutamate Kinase Plos One V. 7 34734 2012.
ISSN: ISSN 1932-6203
PubMed: 22529931
DOI: 10.1371/JOURNAL.PONE.0034734
Page generated: Fri Aug 8 10:19:17 2025
ISSN: ISSN 1932-6203
PubMed: 22529931
DOI: 10.1371/JOURNAL.PONE.0034734
Last articles
I in 6DOQI in 6DOP
I in 6DOO
I in 6DON
I in 6DOM
I in 6DOK
I in 6DOL
I in 6DOJ
I in 6DOH
I in 6DOI