Mercury »
PDB 3wee-4ia4 »
4bjj »
Mercury in PDB 4bjj: SFC1-SFC7 Dimerization Module
Protein crystallography data
The structure of SFC1-SFC7 Dimerization Module, PDB code: 4bjj
was solved by
N.M.I.Taylor,
F.Baudin,
G.Von Scheven,
C.W.Muller,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 33.290 / 2.40 |
| Space group | P 63 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 85.245, 85.245, 154.108, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 18.09 / 22.36 |
Mercury Binding Sites:
The binding sites of Mercury atom in the SFC1-SFC7 Dimerization Module
(pdb code 4bjj). This binding sites where shown within
5.0 Angstroms radius around Mercury atom.
In total 3 binding sites of Mercury where determined in the SFC1-SFC7 Dimerization Module, PDB code: 4bjj:
Jump to Mercury binding site number: 1; 2; 3;
In total 3 binding sites of Mercury where determined in the SFC1-SFC7 Dimerization Module, PDB code: 4bjj:
Jump to Mercury binding site number: 1; 2; 3;
Mercury binding site 1 out of 3 in 4bjj
Go back to
Mercury binding site 1 out
of 3 in the SFC1-SFC7 Dimerization Module
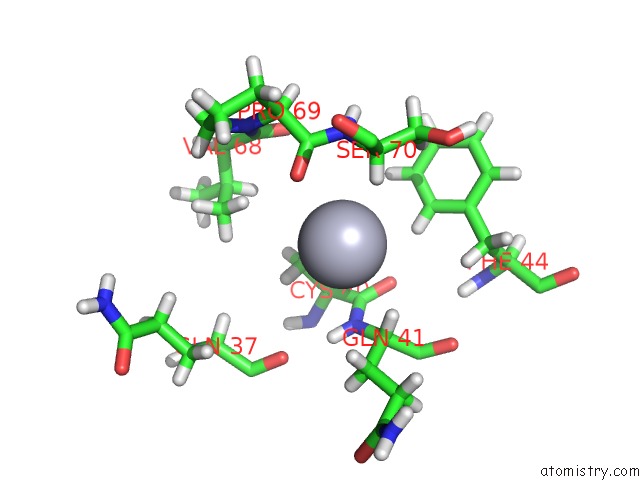
Mono view
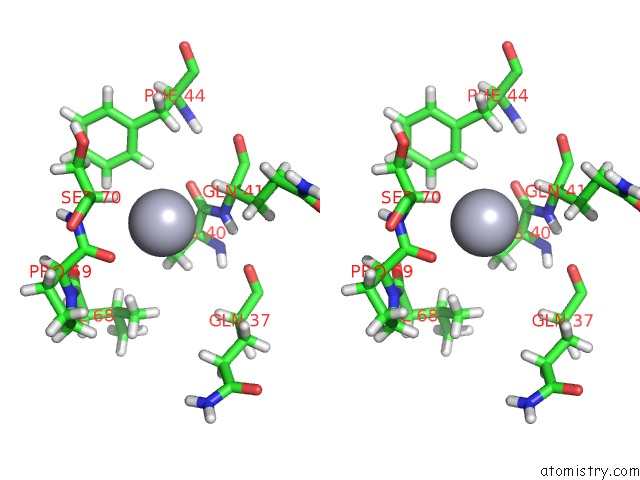
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 1 of SFC1-SFC7 Dimerization Module within 5.0Å range:
|
Mercury binding site 2 out of 3 in 4bjj
Go back to
Mercury binding site 2 out
of 3 in the SFC1-SFC7 Dimerization Module
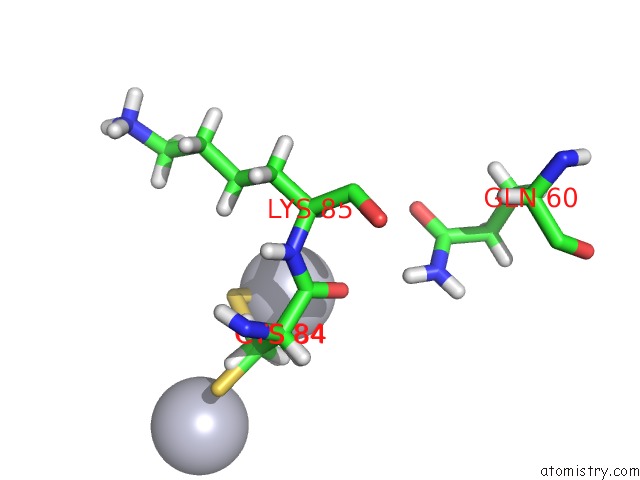
Mono view
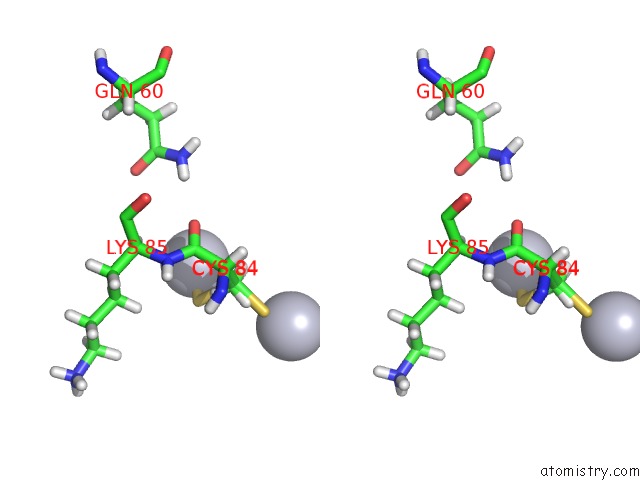
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 2 of SFC1-SFC7 Dimerization Module within 5.0Å range:
|
Mercury binding site 3 out of 3 in 4bjj
Go back to
Mercury binding site 3 out
of 3 in the SFC1-SFC7 Dimerization Module
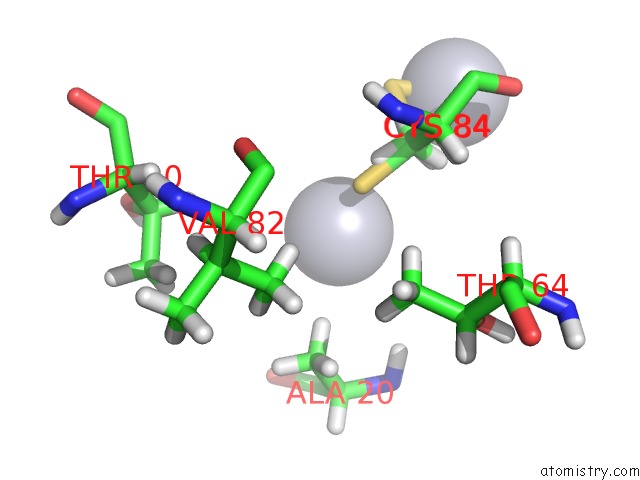
Mono view
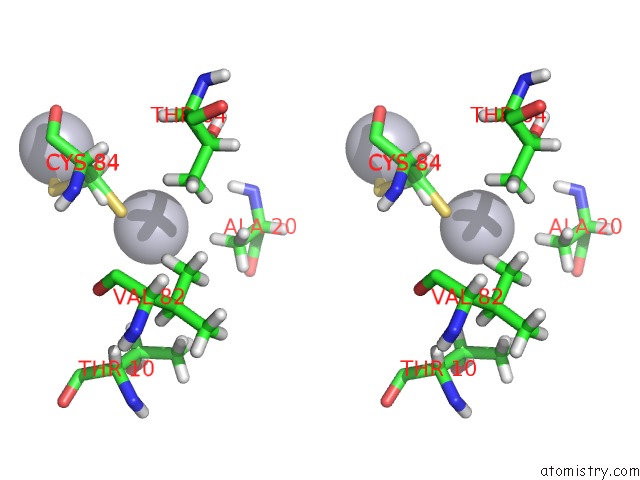
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 3 of SFC1-SFC7 Dimerization Module within 5.0Å range:
|
Reference:
N.M.I.Taylor,
F.Baudin,
G.Von Scheven,
C.W.Muller.
Rna Polymerase III-Specific General Transcription Factor Iiic Contains A Heterodimer Resembling Tfiif RAP30/RAP74. Nucleic Acids Res. V. 41 9183 2013.
ISSN: ISSN 0305-1048
PubMed: 23921640
DOI: 10.1093/NAR/GKT664
Page generated: Sun Aug 11 04:26:25 2024
ISSN: ISSN 0305-1048
PubMed: 23921640
DOI: 10.1093/NAR/GKT664
Last articles
Cl in 2VMCCl in 2VMD
Cl in 2VL4
Cl in 2VM9
Cl in 2VLG
Cl in 2VLI
Cl in 2VKE
Cl in 2VJZ
Cl in 2VKG
Cl in 2VKF