Mercury »
PDB 4q81-5c9l »
4yix »
Mercury in PDB 4yix: Structure of MRB1590 Bound to Adp
Protein crystallography data
The structure of Structure of MRB1590 Bound to Adp, PDB code: 4yix
was solved by
P.L.R.Shaw,
M.A.Schumacher,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 25.60 / 2.60 |
| Space group | C 2 2 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 105.584, 184.709, 73.639, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 20.4 / 26.2 |
Other elements in 4yix:
The structure of Structure of MRB1590 Bound to Adp also contains other interesting chemical elements:
| Magnesium | (Mg) | 3 atoms |
Mercury Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 11;Binding sites:
The binding sites of Mercury atom in the Structure of MRB1590 Bound to Adp (pdb code 4yix). This binding sites where shown within 5.0 Angstroms radius around Mercury atom.In total 11 binding sites of Mercury where determined in the Structure of MRB1590 Bound to Adp, PDB code: 4yix:
Jump to Mercury binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Mercury binding site 1 out of 11 in 4yix
Go back to
Mercury binding site 1 out
of 11 in the Structure of MRB1590 Bound to Adp
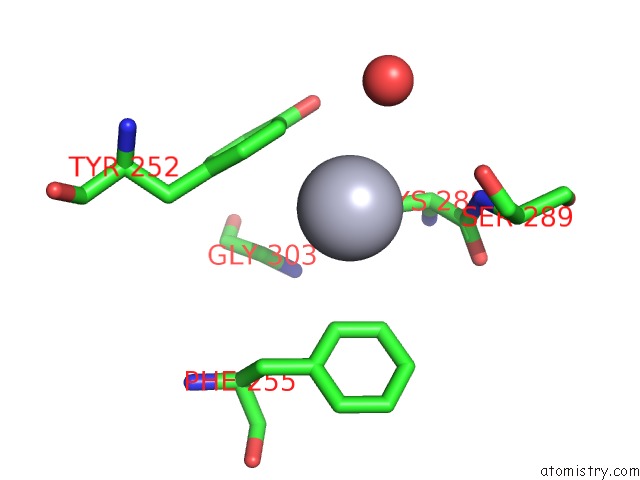
Mono view
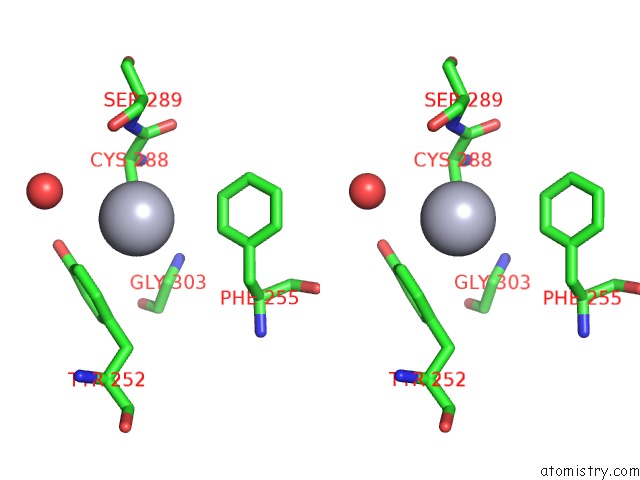
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 1 of Structure of MRB1590 Bound to Adp within 5.0Å range:
|
Mercury binding site 2 out of 11 in 4yix
Go back to
Mercury binding site 2 out
of 11 in the Structure of MRB1590 Bound to Adp
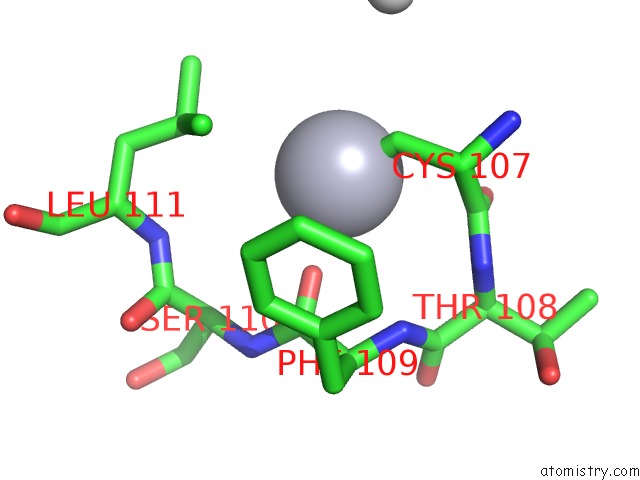
Mono view
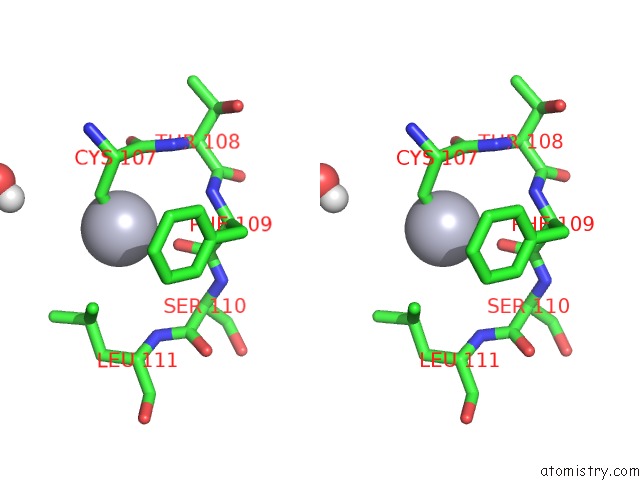
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 2 of Structure of MRB1590 Bound to Adp within 5.0Å range:
|
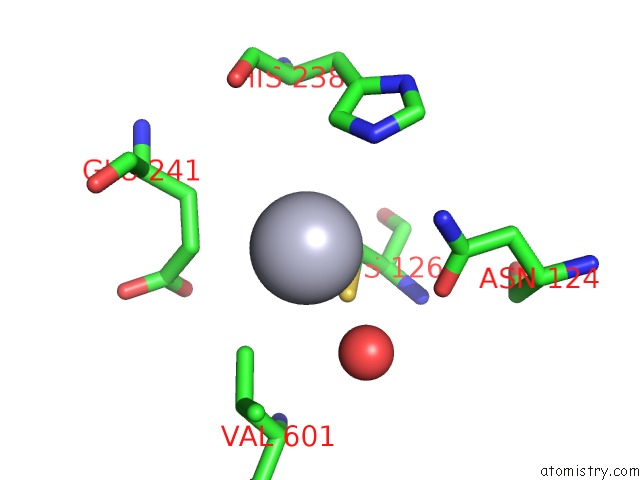
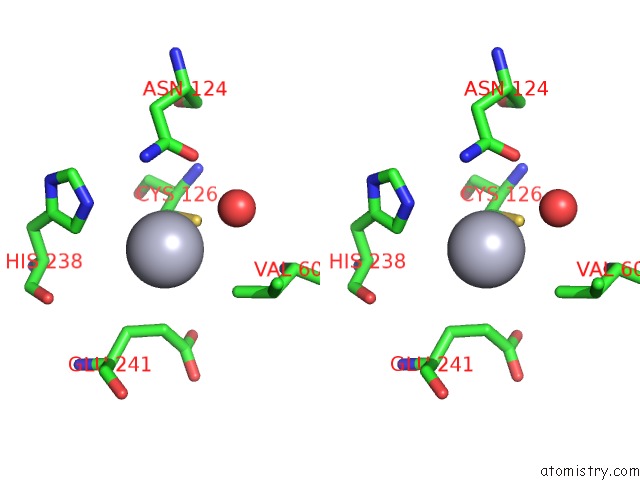
Mercury binding site 3 out of 11 in 4yix
Go back to
Mercury binding site 3 out
of 11 in the Structure of MRB1590 Bound to Adp
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 3 of Structure of MRB1590 Bound to Adp within 5.0Å range:
|
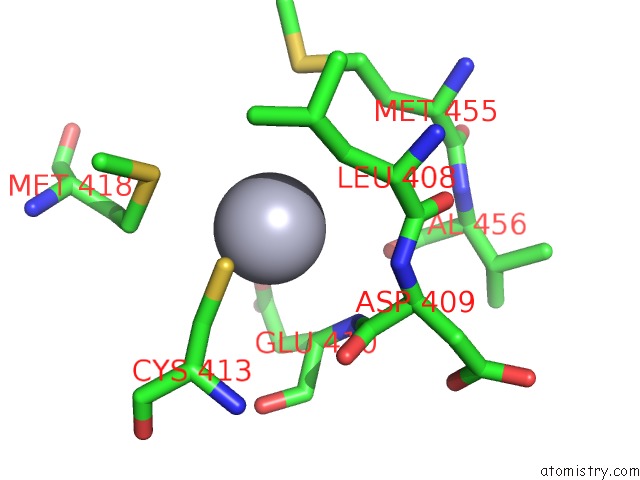
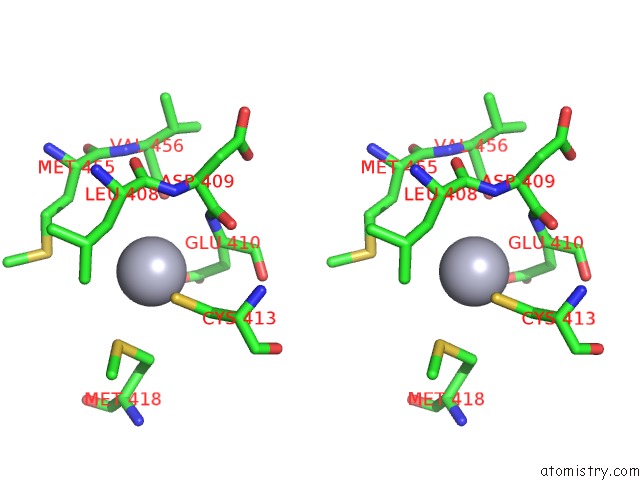
Mercury binding site 4 out of 11 in 4yix
Go back to
Mercury binding site 4 out
of 11 in the Structure of MRB1590 Bound to Adp
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 4 of Structure of MRB1590 Bound to Adp within 5.0Å range:
|
Mercury binding site 5 out of 11 in 4yix
Go back to
Mercury binding site 5 out
of 11 in the Structure of MRB1590 Bound to Adp
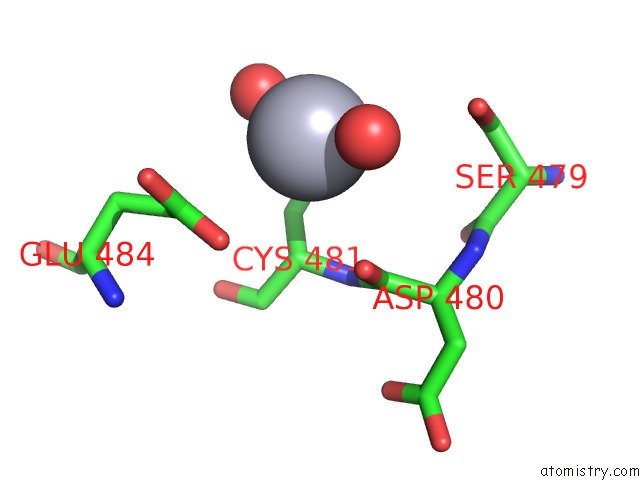
Mono view
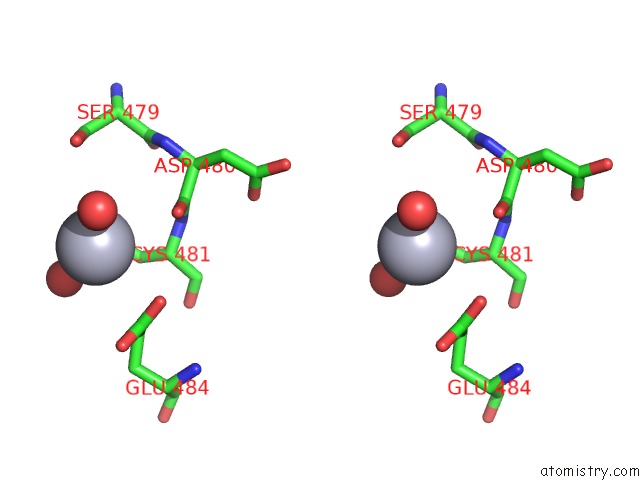
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 5 of Structure of MRB1590 Bound to Adp within 5.0Å range:
|
Mercury binding site 6 out of 11 in 4yix
Go back to
Mercury binding site 6 out
of 11 in the Structure of MRB1590 Bound to Adp
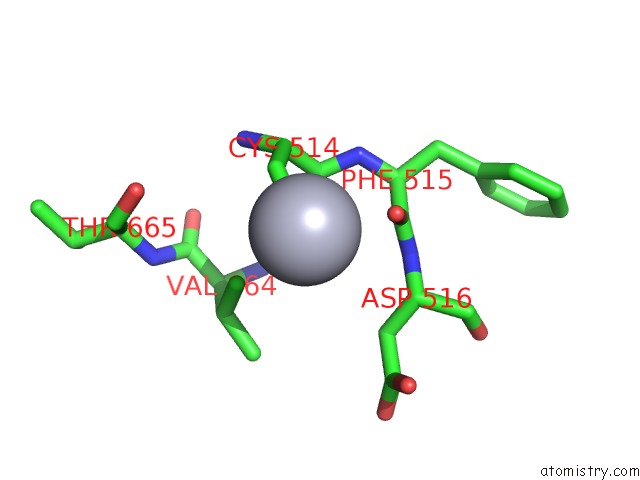
Mono view
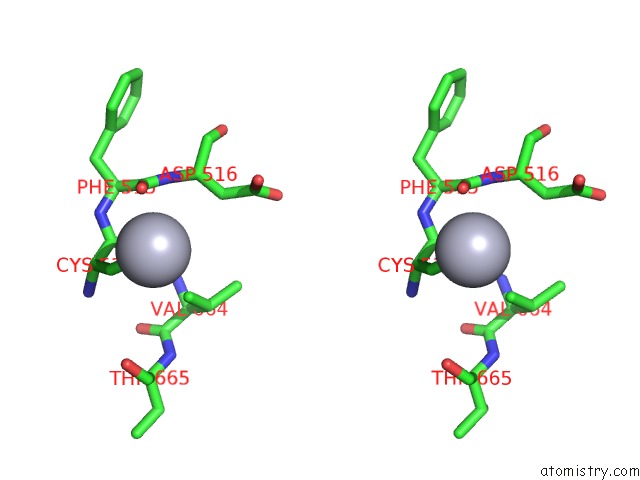
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 6 of Structure of MRB1590 Bound to Adp within 5.0Å range:
|
Mercury binding site 7 out of 11 in 4yix
Go back to
Mercury binding site 7 out
of 11 in the Structure of MRB1590 Bound to Adp
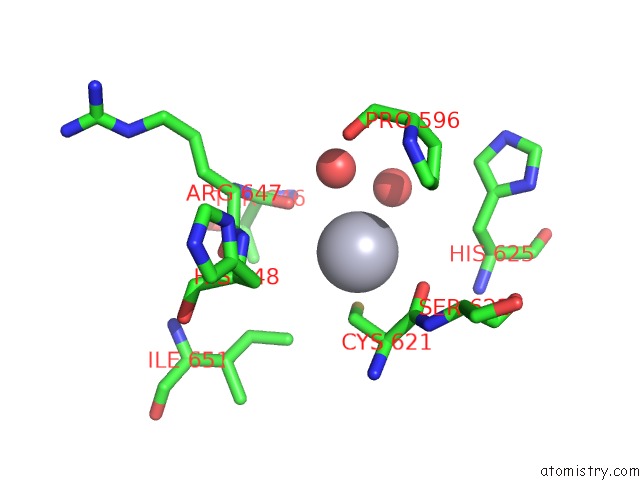
Mono view
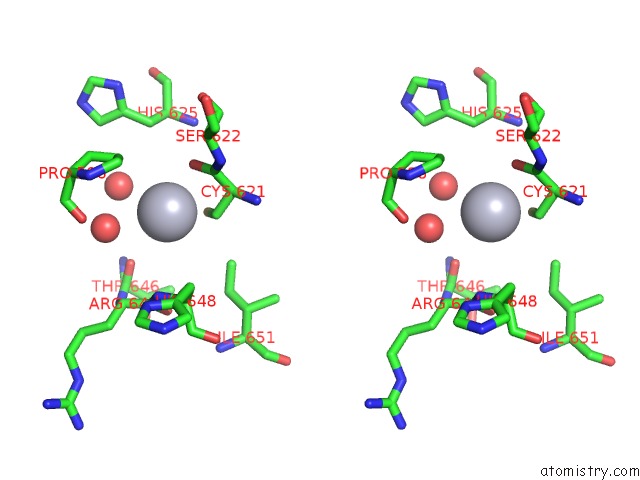
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 7 of Structure of MRB1590 Bound to Adp within 5.0Å range:
|
Mercury binding site 8 out of 11 in 4yix
Go back to
Mercury binding site 8 out
of 11 in the Structure of MRB1590 Bound to Adp
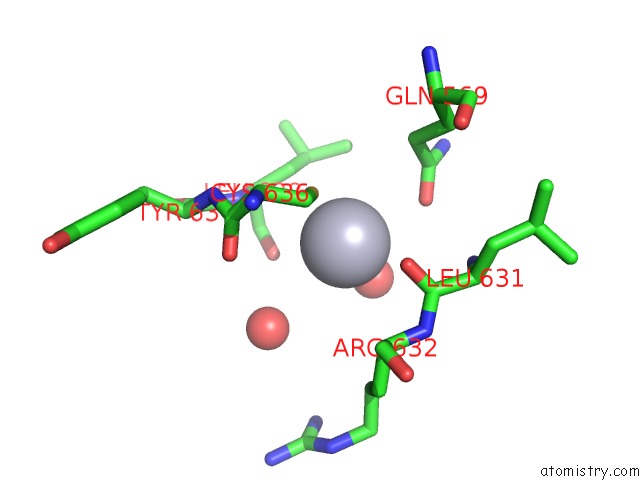
Mono view
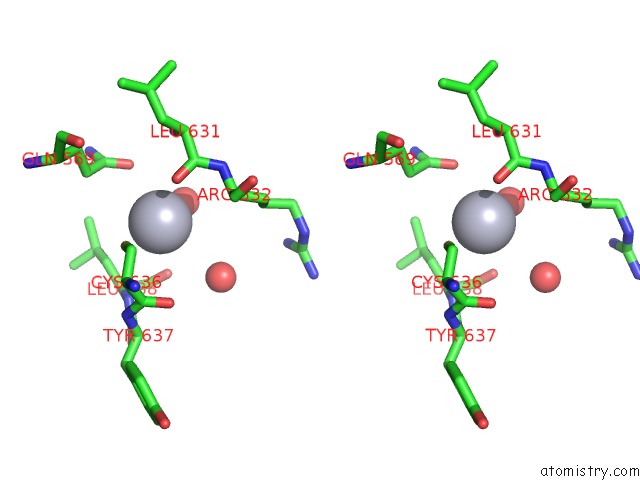
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 8 of Structure of MRB1590 Bound to Adp within 5.0Å range:
|
Mercury binding site 9 out of 11 in 4yix
Go back to
Mercury binding site 9 out
of 11 in the Structure of MRB1590 Bound to Adp
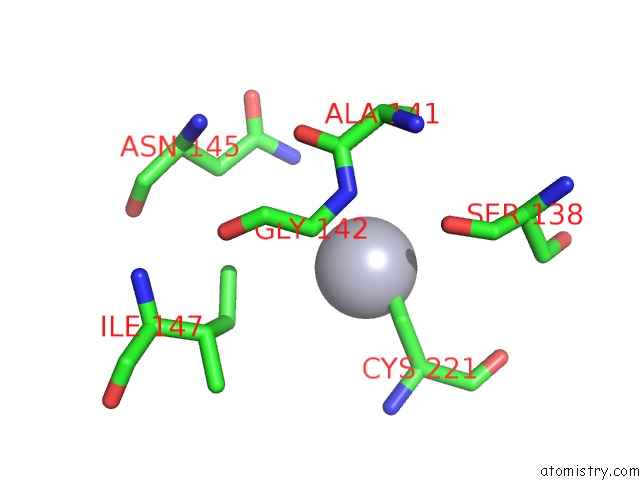
Mono view
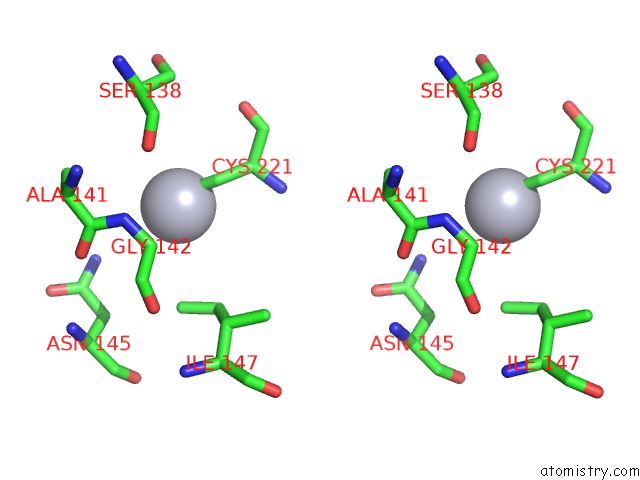
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 9 of Structure of MRB1590 Bound to Adp within 5.0Å range:
|
Mercury binding site 10 out of 11 in 4yix
Go back to
Mercury binding site 10 out
of 11 in the Structure of MRB1590 Bound to Adp
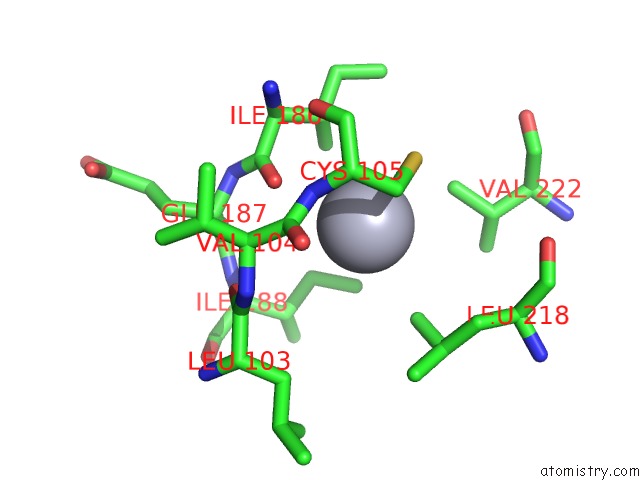
Mono view
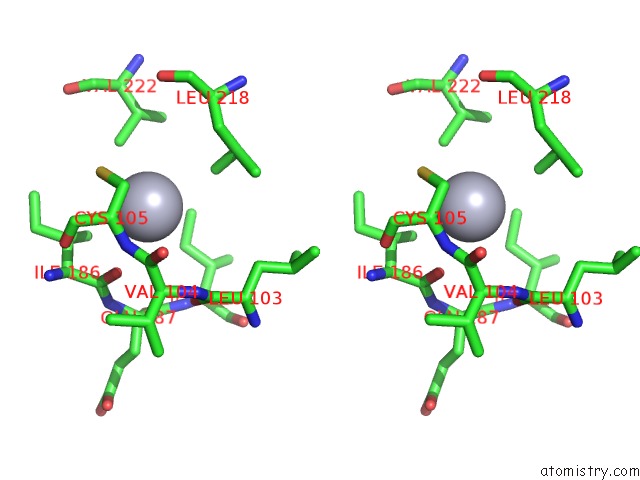
Stereo pair view

Mono view

Stereo pair view
A full contact list of Mercury with other atoms in the Hg binding
site number 10 of Structure of MRB1590 Bound to Adp within 5.0Å range:
|
Reference:
P.L.Shaw,
N.M.Mcadams,
M.A.Hast,
M.L.Ammerman,
L.K.Read,
M.A.Schumacher.
Structures of the T. Brucei Krna Editing Factor MRB1590 Reveal Unique Rna-Binding Pore Motif Contained Within An Abc-Atpase Fold. Nucleic Acids Res. V. 43 7096 2015.
ISSN: ESSN 1362-4962
PubMed: 26117548
DOI: 10.1093/NAR/GKV647
Page generated: Sun Aug 11 05:34:29 2024
ISSN: ESSN 1362-4962
PubMed: 26117548
DOI: 10.1093/NAR/GKV647
Last articles
F in 7NTHF in 7NTI
F in 7NPC
F in 7NRG
F in 7NR5
F in 7NQS
F in 7NOS
F in 7NP5
F in 7NDV
F in 7NP6